Researchers have discovered how a cell surface protein called Applp1 may play a role in spreading the substance that causes Parkinson’s disease from cell to cell in the brain.
What you can expect is an FDA-approved cancer drugs that target another protein called rug 3 – interact with App 1 – stopped its spread in mice, suggesting potential treatments may already exist.
in recent papersan international team of scientists explains how two proteins work together to help harmful substances. alpha-synuclein Protein clumps enter brain cells.
“Now that we know how Applp1 and Lag3 interact, we can better understand how alpha-synuclein contributes to disease progression in Parkinson’s disease,” said Xiaobo Mao, a neuroscientist at Johns Hopkins University. “We’ve found a new method.” said In June.
“Our findings also suggest that targeting this interaction with drugs may significantly slow the progression of Parkinson’s disease and other neurodegenerative diseases.”
Over 8.5 million people Parkinson’s disease exists all over the world. second most common neurodegenerative disease After Alzheimer’s disease.
Because it is a progressive movement disorder, it is usually diagnosed only when symptoms such as tremors, stiffness, balance problems, speech problems, disturbed sleep patterns, and mental health problems appear. Because the disease is currently incurable, patients may eventually find it difficult to walk or talk.
Parkinson’s disease symptoms are primarily caused by the death or failure of dopamine-producing neurons in the brain. substantia nigraan area involved in fine motor control. The possible cause of this is Lewy bodiesThis is an abnormal protein mass consisting primarily of misfolded alpha-synuclein that travels between neurons.
Alpha-synuclein normally maintains functional communication between neurons, but problems arise when it becomes misfolded and insoluble. Having said that, identifying if this is the case, Causes or symptoms of Parkinson’s disease is difficult.
past research About the mouse Discovered that Lag3 binds to α-synuclein protein and spread Parkinson’s disease pathology to neurons. Deletion of Lag3 significantly, but not completely, blocks this process, indicating that other proteins are also involved in neurons uptake of misfolded α-synuclein.
“Our research had previously demonstrated that Lag3 is not the only cell surface protein that helps neurons uptake alpha-synuclein, so we focused on Applp1 in our latest experiments.” said Varina Dawson, a neuroscientist at Johns Hopkins University.
The scientists conducted tests using genetically modified mice lacking either Applp1, Lag3, or both. They found that Applp1 and Lag3 can each independently help brain cells absorb harmful alpha-synuclein, but together they significantly increase uptake.
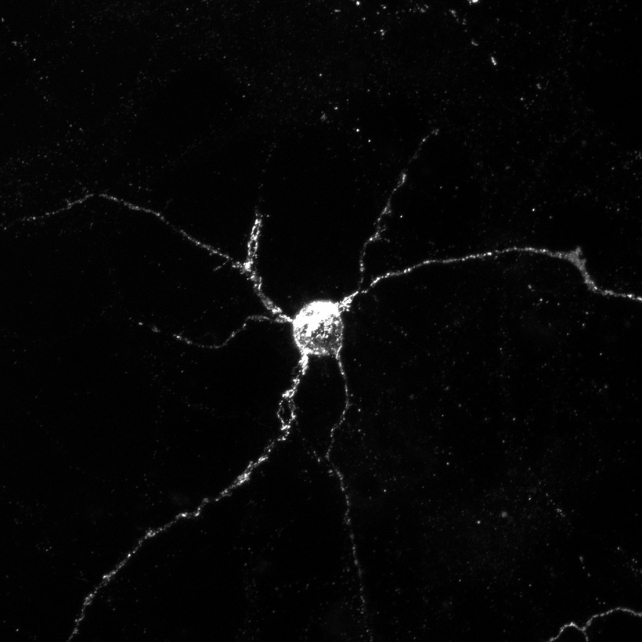
When mice deleted both Applp1 and Lag3, the amount of harmful alpha-synuclein that entered healthy brain cells was reduced by 90 percent. This means that when both proteins were deleted, more of the harmful protein mass was blocked than when only one protein was deleted.
Researchers gave the drug to normal mice Nivolumab/liratlimabmelanoma treatment containing Lag3 antibodyThey found that it also halted the interaction of Applp1 and Lag3, almost completely blocking the formation of alpha-synuclein aggregates that also cause disease in neurons.
“Anti-Lag3 antibodies were successful in preventing further spread of α-synuclein seeds in a mouse model and showed better efficacy than Lag3 depletion, as Applp1 is closely associated with Lag3.” said Ted Dawson, neuroscientist at Johns Hopkins University.
The next step is to test Lag3 antibodies in mouse models of Parkinson’s and Alzheimer’s disease. Research points to Lag3 Also as a target.
This research nature communications.
A previous version of this article was published in June 2024.










