When Dan Heller acquired his first batch of Dexcom’s newest steady glucose displays in early 2023, he determined to run a small experiment: He wore the brand new biosensor and the earlier technology on the identical time to see how they in contrast in measuring his glucose ranges.
The brand new, seventh-generation mannequin (aptly known as the G7) made by San Diego-based healthcare firm Dexcom had simply begun delivery within the United States. Dexcom claimed the G7 to be the “most correct sensor” accessible to the hundreds of individuals with Sort 1 diabetes who use steady glucose displays to assist handle their blood sugars. However Heller discovered that its real-world efficiency wasn’t as much as par. In a September 2023 put up on his Substack, which is devoted to overlaying Sort 1 diabetes analysis and administration, he wrote in regards to the expertise and predicted a rise in adversarial occasions with the G7, drawing on his previous expertise main tech and biotech corporations.
Within the two years since Heller’s experiment, many different customers have reported points with the machine. Some complaints regard failed connection and deployment points, which Dexcom claims to have now addressed. Extra regarding are experiences of erratic, inaccurate readings. A public Fb group devoted to sharing detrimental experiences with the G7 has grown to hundreds of customers, and a number of other class motion lawsuits have been filed towards the corporate, alleging false promoting and deceptive claims about machine accuracy.
But, based mostly on a regular metric within the business, the G7 is without doubt one of the most correct glucose sensors accessible. “Accuracy within the efficiency of our machine is our primary precedence. We perceive it is a lifesaving machine for folks with Sort 1 diabetes,” Peter Simpson, Dexcom’s senior vp of innovation and sensor know-how, informed IEEE Spectrum. Simpson acknowledged some variability in particular person sensors, however stood by the accuracy of the gadgets.
So why have customers confronted points? Partially, metrics utilized in advertising may be deceptive in comparison with actual world efficiency. Variations in research design, mixed with advanced organic realities, imply that the accuracy of those biosensors can’t be boiled down to at least one quantity—and customers are studying this the arduous approach.
Dexcom’s Glucose Displays
Steady glucose displays (CGMs) usually encompass a small filament inserted below the pores and skin, a transmitter, and a receiver. The filament is coated with an enzyme that generates {an electrical} sign when it reacts with glucose within the fluid surrounding the physique’s cells. That sign is then transformed to a digital sign and processed to generate glucose readings each couple of minutes. Every sensor lasts per week or two earlier than needing to get replaced.
The know-how has come a great distance in recent times. Within the 2010s, these gadgets required blood glucose calibrations twice a day and nonetheless weren’t dependable sufficient to dose insulin based mostly on the readings. Now, some insulin pumps use the near-real-time information to mechanically make changes. With these enhancements has come higher belief within the information customers obtain—and better requirements. A defective studying might end in a harmful dose of insulin.
The G7 launched a number of adjustments to Dexcom’s earlier designs, together with a a lot smaller footprint, and up to date the algorithm used to translate sensor alerts into glucose readings for higher accuracy, Simpson says. “From a efficiency perspective, we did exhibit in a scientific trial that the G7 is considerably extra correct than the G6,” he says.
So Heller and others have been stunned when the brand new Dexcom sensor appeared to be performing worse. For some batches of sensors, it’s potential that the problem was partially on account of an unvalidated change in a element utilized in a resistive layer of the sensors. The brand new element confirmed worse efficiency, in line with a warning letter issued by the U.S. Meals and Drug Administration in March 2025, following an audit of two U.S. manufacturing websites. The fabric has since been faraway from all G7 sensors, Simpson says, and the corporate is continuous to work with the FDA to deal with issues. (“The warning letter doesn’t limit Dexcom’s capability to provide, market, manufacture or distribute merchandise, require recall of any merchandise, nor limit our capability to hunt clearance of latest merchandise,” Dexcom added in an announcement.)
“There’s a distribution of accuracies that must do with folks’s physiology and in addition the gadgets themselves. Even in our scientific research, we noticed some that have been actually exact and a few that had just a little little bit of inaccuracy to them,” says Simpson. “However typically, our sensor could be very correct.”
In late November Abbott—one in every of Dexcom’s fundamental rivals—recalled a few of its CGMs on account of inaccurate low glucose readings. The recall impacts roughly 3 million sensors and was brought on by a problem with one in every of Abbott’s manufacturing strains.
The discrepancy between reported accuracy and consumer expertise, nonetheless, goes past anyone firm’s manufacturing missteps.
Does MARD Matter?
The accuracy of CGM programs is steadily measured by way of “imply absolute relative distinction,” or MARD, a proportion that compares the sensor readings to laboratory blood glucose measurements. The decrease the MARD, the extra correct the sensor.
This quantity is usually utilized in promoting and advertising, and it has a historic relevance, says Manuel Eichenlaub, a biomedical engineer on the Institute for Diabetes Expertise Ulm in Germany, the place he and his colleagues conduct impartial CGM efficiency research. For years, there was a common perception {that a} MARD below 10 % meant a system could be correct sufficient for use for insulin dosing. In 2018, the FDA established a selected set of accuracy necessities past MARD for insulin-guiding glucose displays, together with Dexcom’s. However producers design the scientific trials that decide accuracy metrics, and the best way research are designed could make an enormous distinction.
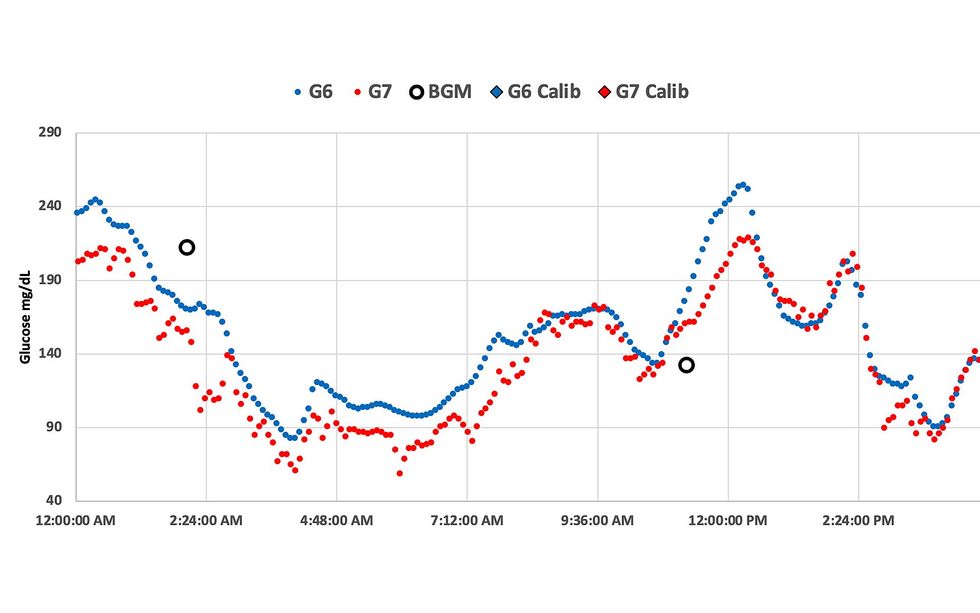 When Dan Heller wore the Dexcom G6 and G7 on the identical time, he says he seen the G7 readings have been extra erratic, making it harder to correctly management his blood sugar. Dan Heller
When Dan Heller wore the Dexcom G6 and G7 on the identical time, he says he seen the G7 readings have been extra erratic, making it harder to correctly management his blood sugar. Dan Heller
As an example, blood glucose ranges function the “floor fact to match the CGM values towards,” says Eichenlaub. However glucose ranges fluctuate throughout blood compartments within the physique; blood collected from capillaries with a finger prick fluctuates extra and might have glucose ranges round 5 to 10 % larger than venous blood. (Dexcom exams towards a gold-standard venous blood analyzer. When customers see inaccuracies towards dwelling meters that use capillary blood, it might partially be a mirrored image of the meter’s personal inaccuracy, Simpson says, although he acknowledges actual inaccuracies in CGMs as effectively.)
Moreover, the distribution of sampling isn’t standardized. CGMs are identified to be much less correct at the start and finish of use, or when glucose ranges are out of vary or altering shortly. Which means measured accuracy could possibly be skewed by taking fewer samples proper after a meal or late within the CGM’s lifetime.
In keeping with Simpson, Dexcom’s trial protocol meets the FDA’s expectation and exams the gadgets in numerous blood sugar ranges throughout the lifetime of the sensor. “Inside these scientific trials, we do stress the sensors to try to simulate these actual world situations,” he says.
Dexcom and different corporations promote a MARD round 8 %. However some impartial research are extra demanding and discover larger numbers; a head-to-head research of three fashionable CGMs that Eichenlaub led discovered MARD values nearer to 10 % or larger.
Eichenlaub and different CGM consultants imagine that extra standardization of testing and an extension of the FDA necessities are crucial, in order that they lately proposed complete pointers on CGM efficiency testing. In america and Europe, just a few producers presently dominate the market. However newer gamers are getting into the rising market and, particularly in Europe, might not meet the identical requirements as legacy producers, he says. “Having a standardized approach of evaluating the efficiency of these programs is essential.”
For customers like Heller although, higher accuracy solely issues if it yields higher diabetes administration. “I don’t care about MARD. I need information that’s reliably actionable,” Heller says. He encourages engineers engaged on these gadgets to assume just like the affected person. “In some unspecified time in the future, there’s quantitative information, however you want qualitative information.”
From Your Website Articles
Associated Articles Across the Internet

